Urgent Contact
Whatsapp: +8615524105871
+8613042442971
Due to the impact of corona virus and our large order quantity, please contact us via whatsapp to confirm stock and price in real time!
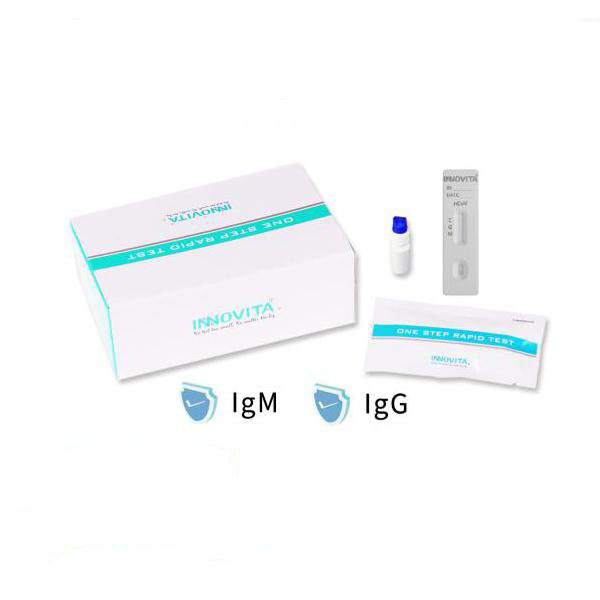
Product Name
2019-nCoV Ab Test (Colloidal Gold)
Intended Use
The kit is intended for the qualitative detection of IgM and IgG antibodies against 2019 Novel Coronavirus (2019-nCoV) in human serum/plasma/venous whole blood specimen and for the auxiliary diagnosis of 2019-nCoV infection.
Summary
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Serve Acute Respiratory Syndrome (SARA-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously indentified in humans.
Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death.
Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
A novel coronavirus (CoV) is a new strain of coronavirus that has not been previously identified in humans. The new, or "novel" coronavirus, now called 2019-nCoV, had not been previously defected before the outbreak ws reported in Wuhan, China in December 2019.
Current estimates of the incubation period range from 1-12.5 days with median estimates of 5-6 days. These estimates will be refined as more data becomes available. Based on information from other coronavirus diseases, such as MERS and SARS, the incubation period of 2019-nCoV could be up to 14 days. WHO recommends that the follow-up of contacts of confirmed cases is 14 days.
To date, there is no specific medicine recommended to prevent or treat the novel coronavirus.
Principle
The kit detects 2019-nCoV IgM and IgG antibodies by immuno-capture method. The nitrocellulose membrane is coated by mouse-anti human monoclonal IgM antibodies, mouse-anti human monoclonal IgG antibodies, and goat-anti-mouse IgG antibodies. The recombinant 2019-nCoV antigen and mouse IgG antibodies are labeled with colloidal gold as a tracer. After addition of the specimens, if 2019-nCoV IgM antibodies are present, the antibodies will bind to colloidal gold-coated 2019-nCoV antigens to form compounds, which are further captured by pre-coated mouse-anti human IgM antibodies to form new compounds, and generate purple line (M). If 2019-nCoV IgG antibodies are present in specimen, the antibodies will bind to colloidal gold-labeled 2019-nCoV antigens to form compounds and further form new compounds by binding to pre-coated mousi-anti human monoclonal IgG antibodies, which give rise to purple line (G). The binding of colloidal gold-labeled mouse IgG antibodies with goat-anti-mouse IgG antibodies will present purple line, which is used as the control line (C).
Composition
1. Sealed foil pouches each containing:
a. One cassette device
b. One desiccant
2. Specimen diluent
3. Instructions for use
Storage and Stability
1. Store at 4℃~30℃ (39.2℉~86℉)
2. Use the test within 1 hour after opening the pouch under 60% humidity.
3. See production date and expiration date on label.
Specimen Collection and Handling
Consider any materials of human origin as infectious and handle them using standard bio-safety procedures.
1. The kit is intended for test only in serum/plasma/venous whole blood specimens.
2. Specimens should be collected by standard protocol.
3. The venous whole blood specimens could be stored at 2℃~8℃ (36℉~46℉) for up to 3 days, and it couldn't be frozen. Venous whole blood specimens can be anti-coagulated with routine dosage of heparin (9.8-28IU/mL), sodium citrate (3.8%, equivalent to 129mmol/L), Ethylene Diamine Tetraacetic Acid (EDTA) (4.55MMOL/ml±0.85mmol/ml).
4. The serum or plasma specimens could be stored at 2℃~8℃(36℉~46℉) for up to 7 days, and could be frozen at -20℃(-4℉) for 6 months. The specimens are repeated frozen and thawed no more than 8 times; it should be the best to test the sample after collection immediately.
5. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible matter should be clarified by centrifugation before testing.
Test Procedure
1. Allow the best, specimen diluent and/or controls to reach room temperature 10℃~30℃ (50℉~86℉) prior to testing.
2. Remove the test device from the sealed pouch and use it as soon as possible.
3. Place the test device on a clean and level surface.
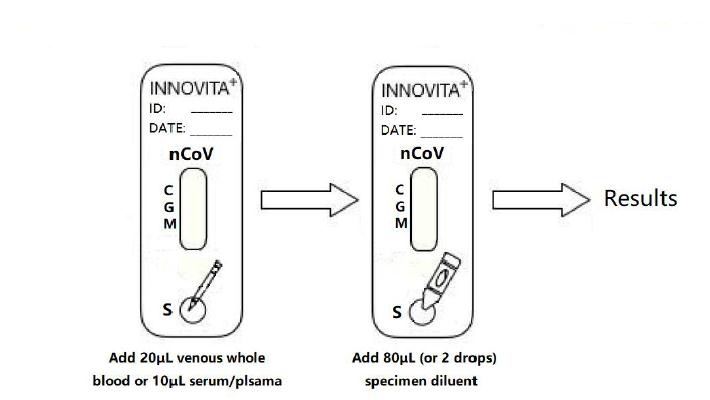
4. FROM THE TOP OF THE SPECIMEN WELL: Add 20μL venous whole blood or 10μL serum/plasma specimen into the specimen well.
5. FROM THE BOTTOM OF THE SPECIMEN WELL: Add 80μL or 2 drops of specimen diluent into the specimen well.
6. Wait for the colored line(s) to appear. Read results within 15 minutes. Do not read the after 15 minutes.
Results Interpretation
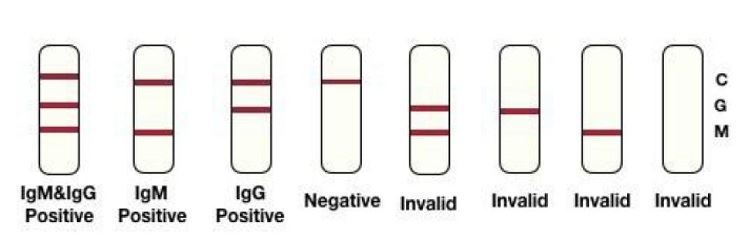
1. IgM Positive: The presence of two purple bands (M and C) indicates positive for 2019-nCoV IgM antibodies.
2. IgG Positive: The presence of two purple bands (G and C) indicates positive for 2019-nCoV IgG antibodies.
3. IgM & IgG Positive: If the C line and both M and G line develop, it indicates positive for both 2019-nCoV IgM and IgG antibodies.
4. Negative: Only one purple band appearing at the control line (C) indicates negative result.
5. Invalid: If control line (C) fails to appear, no matter whether the G/M line is visible or not, the best is invalid. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a test device. If the Problem persists, you should immediately stop using the kit with the same LOT No. and contact your local distributor.
Performance Characteristics
1. Use the national or enterprise reference controls for testing, and the results meet the detection requirements of national or enterprise reference controls.
2. Test the samples with a titer of 1:320 at the original concentrations with the 2019-nCoV IgM antibody and 2019-nCoV IgG antibody. No hook Effect was observed.
3. The clinical trial of this product is based on the clear diagnosis/exclusion criteria of the disease identified in the "Novel Corona virus Pneumonia Diagnosis and Treatment Program". Clinical research was conducted in 5 institutions and the total cases were 44.7. Using this kit, 110 cases out of 126 clinically confirmed cases are positive, with the sensitivity of 87.3% (95% CI: 80.40% to 92.0%); 62 cases of clinically excluded cases are totally negative with the specificity of 100% (95 Cl: 94.20% to 100%).
4. Avoid using special samples: red background may appear in the hyperlipemia (triglyceride concentration higher than 25mg/ml), icteric samples (Bilirubin concentration higher than 0.2mg/ml) and hemolytic specimen (hemoglobin concentration more than 5.0mg/ml). which may affect the test result.
5. The 2019-nCoV IgM test was also evaluated with samples that are IgM positive for other diseases as listed in the following table. No cross reactivity was observed.
| Coronavirus HKU1-IgM | Coronavirus OC43-IgM |
| Coronavirus NL63-IgM | Coronavirus 229E-IgM |
| Influenza A virus H1N1 (new type influenza A virus H1N1 2009, seasonal influenza virus H1N1) IgM | H3N2-IgM |
| H5N1-IgM | H7N9-IgM |
| Influenza B virus IgM | Respiratory Syncytial Virus IgM |
| Adenovirus IgM | Rhinovirus IgM |
| Enterovirus A-IgM | EB virus IgM |
| Measlea virus IgM | Cytomegalovirus IgM |
| Rotavirus IgM | Mumps IgM |
| Varicella-zoster virus IgM | Parainfluenza virus IgM |
| Mycoplasma pneumoniae IgM | Chlamydia pneumoniae IgM |
| Coxsackievirus group B IgM |
6. The 2019-nCoV IgG test was also evaluated with samples that are IgG positive for other diseases as listed in the followig table. No Cross reactivity was observed.
| Coronavirus HKU1-IgG | Coronavirus OC43-IgG |
| Coronavirus NL63-IgG | Coronavirus 229E-IgG |
| Influenza A virus H1N1 (new type influenza A virus H1N1 2009, seasonal influenza virus H1N1) IgG | H3N2-IgG |
| H5N1 -IgG | H7N9-IgG |
| Influenza B virus IgG | Respiratory Syncytial Virus IgG |
| Adenovirus IgG | Rhinovirus IgG |
| Enterovirus A-IgG | EB virus IgG |
| Measles virus IgG | Cytomegalovirus IgG |
| Rotavirus IgG | Mumps IgG |
| Varicella-zoster virus IgG | Parainfluenza virus IgG |
| Mycoplasma pneumoniae IgG | Chlamydia pneumoniae IgG |
| Coxsackievirus group B IgG |
7. RF, ANA and AMA don't exhibit cross reactivity with the test.
8. Common antivirals such like Epistine hydrochloride (≤4mg/L), Ribavirin (≤40mg/L), Interferon (≤200mg/L), Oseltamivir (≤30mg/L), Abidol (≤40mg/L), Levofloxacin (≤200mg/L), Azithromycin (≤100mg/L), Ceftriaxone (≤ 400mg/L), Meropenem (≤200mg/L) have to interference effect on the detection of this kit.
9. Systemic lupus erythematosus has no interference effect on the detection of this kit.
10.Non-specific IgM antibody (≤0.8mg/ml) and non-specific IgG antibody (≤4mg/ml) have to interference effect on the detection of this kit.
11. Heparin, sodium citrate, EDTA and other anticoagulants have no interference effect on the detection of this kit.
12. The precision experiments were carried out by different experimenters, at different times and at different places, and the results met the product performance requirements.
13. After the specific IgM positive sample was destroyed by β-mercaptoethanol, the IgM test result was negative.
14. After preliminary evaluation, it is basically confirmed that the clinical performance of the product can meet the emergency needs of the epidemic. The product will further collect clinical data to confirm the clinical performance of the product after it is marketed.
Limitations
1. The kit is for qualitative detection and aid diagnosis use only.
2. In the early phase of infection, no IgG or IgM antibody will be produced, or the titer will be very low, thus, negative result will occur. Re-testing will be conducted in 7-14 days, and the sample that is collected last time will be detected in parallel during re-testing to confirm whether the serology turns positive or the titer increases significantly.
3. The reference value of serological antibody defection is limited for the immune-compromised patients or patients who receive immunosuppressive therapy.
4. Positive IgM antibody test will occur not only in primary infection, but also in secondary infection.
5. Positive IgG test indicates previous infection or secondary infection.
6. The confirmation or exclusion of infection will be combined with the patient's clinical manifestations or further other methods.
Precaution
1. Use fresh specimens whenever possible.
2. Results read after 15 minutes are considered invalid.
Certificate
